Cysteine: The Best Amino Acid
Before we begin discussing the magic that is cysteine, let’s briefly discuss what, exactly, an amino acid is:
The name “amino acid” comes from the commonly found structures in an amino acid: “amines”, which are a nitrogen bonded to two hydrogens, and “carboxylic acid functional groups”, which are a carbon double bonded to an oxygen and single-bonded to a hydroxide. Amino acids are molecules that are incredibly significant to life. This is because they play a key role in building proteins in your body. Proteins are necessary in your body to make things from muscle tissue to haemoglobin – without them we wouldn’t be alive!
However, today we’re going to learn about my favourite amino acid, cysteine. Cysteine looks a little something like this:
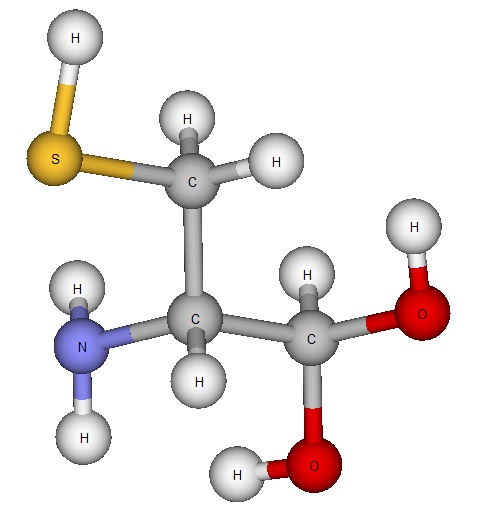
Cysteine’s molecular formula is C3H7NO2S. It is one of the two amino acids that contain sulphur and it is very physically similar to the amino acid serine. But why is cysteine important? Cysteine is critical to the stabilization and structure of proteins due to its reactive sulphurs which form something called “disulfide bridges”. These bridges make polypeptide chains – chains of amino acids – much more stable and form a sort of “loop backbone” on molecules. The quaternary structure of many proteins exists thanks to cysteine and its disulphide bridges, and disulphide bridges play a key role in all of the other structures as well. Cysteine is also the only amino acid which has a highly reactive sulfhydryl group – the group that makes the disulfide bridges possible - making it irreplaceable.
Cysteine doesn’t just help you build proteins, though. Cysteine is a jack-of all-trades. It donates sulphides to your metabolism process, allowing you to convert food into energy. It reacts with sugars and heat to brown meat and produce the flavour you taste when you eat it. Due to its role in polypeptide chains, it is a key component in keratin, the structural proteins that make up your nails, your hair, and the outermost layer of your skin. In animals, keratin helps create horns, hooves, claws, scales, beaks, feathers – all kinds of useful things! It even makes up the wool that sheep produce, and the baleen plates in the mouths of filter-feeding whales. Possibly the best part about cysteine? It’s a non-essential amino acid, meaning that your body makes it all by itself. It does, however, need enough methionine to take sulphurs from – but methionine can be found in things like eggs, sesame seeds, chicken, fish, beef, bacon, peanuts, and cereals.
Can you imagine birds without feathers and beaks? Horses without hooves? Can you imagine never knitting, or throwing on that cozy wool sweater? Can you imagine not having skin, nails, or hair? Most importantly, can you imagine having unstable proteins, among other things? You’d best thank cysteine – it works hard to create the world around you, and you yourself!
Sources:
http://www.biology.arizona.edu/biochemistry/problem_sets/aa/aa.html
http://www.cryst.bbk.ac.uk/pps97/assignments/projects/leluk/project.htm#Fig2
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2386534/
(I’ll also mention that a LOT of the information came from my biology class/notes, and that the molecular model included was made by myself via a program called KnowItAll)